Insight into Debye Hückel length (κ−1): smart gravimetric and swelling techniques reveals discrepancy of diffuse double layer theory at high ionic concentrations | SpringerLink

Rigorous treatment of pairwise and many-body electrostatic interactions among dielectric spheres at the Debye–Hückel level | SpringerLink

Insight into Debye Hückel length (κ−1): smart gravimetric and swelling techniques reveals discrepancy of diffuse double layer theory at high ionic concentrations | SpringerLink
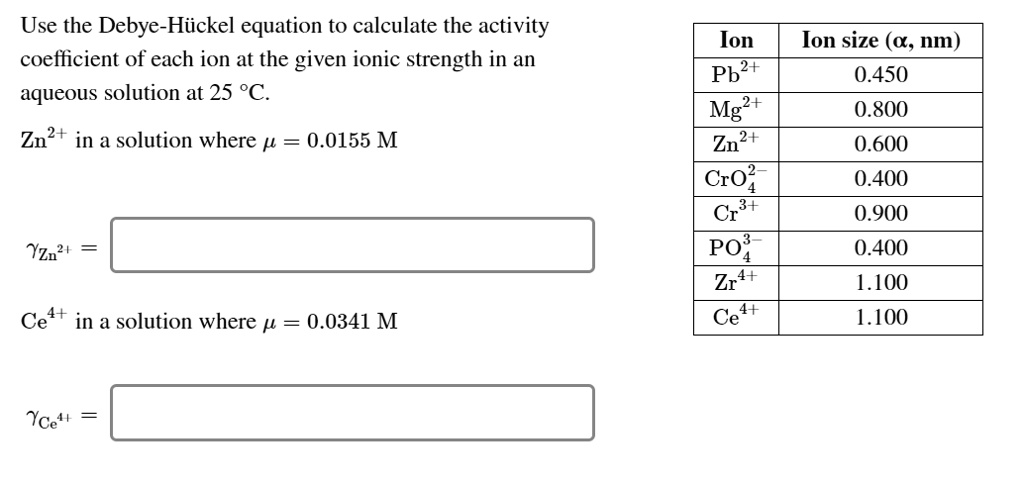
SOLVED: Use the Debye-Hiickel equation to calculate the activity coefficient of each ion at the given ionic strength in an aqueous solution at 25 *C. Ion Pb?+ Mg2+ Zn2+ Cr0? Cr PO
The theory of electrolytes. I. Freezing point depression and related phenomena' (Debye & Hückel, 1923)